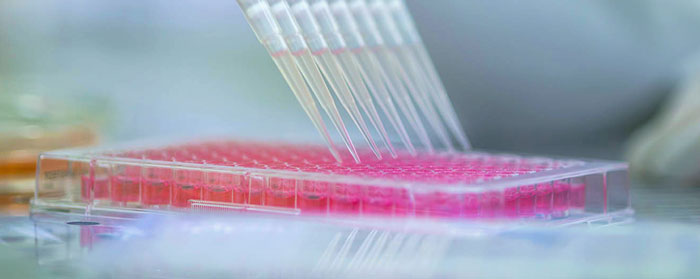
Diagnostic tests development
Your partner from product development to regulatory compliance
We can bring your research to application. We leverage our development and industrialization resources and talents to turn your idea into a successful product. This service also applies to diagnostic tests that already exist but that can be improved to increase performance, for example by adopting automated and high-throughput platforms or combining the detection of several analytes. Validation of assay performance and regulatory support for commercialization purposes can be provided as well.
Custom antibodies and proteins
Our goal is to provide a flexible antibody and protein service program tailored to your needs
Our clients are research laboratories and institutions and commercial companies. The final goal is the same: a fast track for your scientific discoveries. DIATHEVA is the resourceful partner that makes you fill the gap between your application and the lack of research tools for it. We offer you a fast and cost-efficient customised production without compromising on top quality and high lot-to-lot reproducibility.
Custom antibodies and proteins
Our goal is to provide a flexible antibody and protein service program tailored to your needs
Our clients are research laboratories and institutions and commercial companies. The final goal is the same: a fast track for your scientific discoveries. DIATHEVA is the resourceful partner that makes you fill the gap between your application and the lack of research tools for it. We offer you a fast and cost-efficient customised production without compromising on top quality and high lot-to-lot reproducibility.
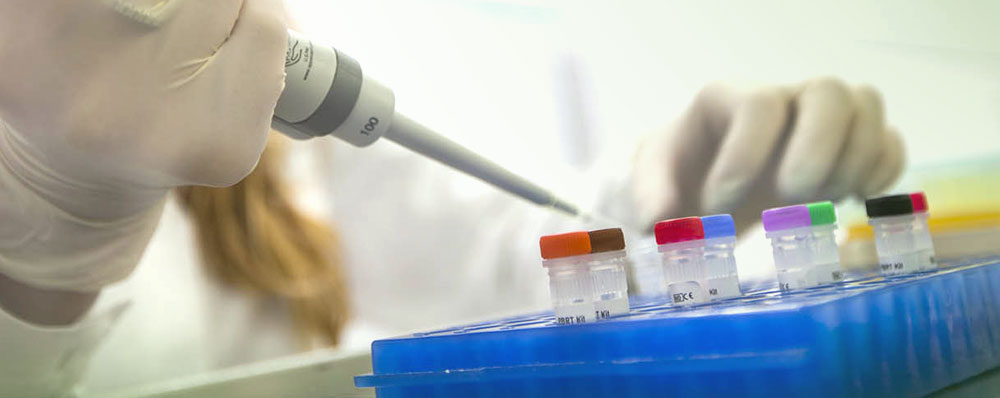
Kit development
Custom R&D and manufacturing of diagnostic kits – we make it your way
Diatheva has invested resources for the creation of new laboratory tests and for the continuous improvement of our products in collaboration with strategic synergies and many partners. In close collaboration with our customers, we identify the ideal product concept and develop it into a market-ready product according to specific requirements, establishing a robust and reproducible manufacturing process for your product. We offer quality, consistency and validation activities following international standards.

Design and feasibility study

Development and Validation
Manufacturing

Compliance
Kit development
Custom R&D and manufacturing of diagnostic kits – we make it your way
Diatheva has invested resources for the creation of new laboratory tests and for the continuous improvement of our products in collaboration with strategic synergies and many partners. In close collaboration with our customers, we identify the ideal product concept and develop it into a market-ready product according to specific requirements, establishing a robust and reproducible manufacturing process for your product. We offer quality, consistency and validation activities following international standards.

Design and feasibility study

Development and Validation

Manufacturing

Compliance
Regulatory support
We will guide you through the regulatory maze to commercialize your diagnostic test
We assist you in the design and preparation of the technical files necessary to submit the application for CE Marking In Vitro Diagnostic (IVD) which is required for all diagnostics tests before commercialization. Also, we can facilitate the regulatory submission for your products that are already CE-IVD certified, which will need to be updated according to the new normative IVDR 2017/746, coming into force in 2022.

Regulatory support
We will guide you through the regulatory maze to commercialize your diagnostic test
We assist you in the design and preparation of the technical files necessary to submit the application for CE Marking In Vitro Diagnostic (IVD) which is required for all diagnostics tests before commercialization. Also, we can facilitate the regulatory submission for your products that are already CE-IVD certified, which will need to be updated according to the new normative IVDR 2017/746, coming into force in 2022.
